
The Origins of Kambô
Each tribe has its own legend or story about how they came to use Kambô. The most prevalent legend comes from Brazil…
This Huni Kuin (Kaxinawá) legend tells that the Indians of the tribe were very ill and their medicine man (Pajé in Brazil) had done everything that was possible to cure them. All medicinal herbs known were used, but none helped.
Under the effect of sacred plant medicines, he entered the forest and whilst there received a visit from a female spirit of the forest.
She brought in her hands a frog, from which she took a white secretion, and taught the Pajé how to apply it. Returning to the tribe and following the guidelines he had received, the Pajé was able to cure his brothers and sisters. From then on he was known as Pajé Kampu or Kampum.
After his death, his spirit lived on in the frog, where it continued its mission to protect the health of those who defend the forest. The secretion became known as Kambô, but in some tribes it is called Sapo, Dow-Kiet, Kampu or Vacina da Floresta.
Usage spread, and for thousands of years, Kambô has been used as medicine by the Huni Kuin (Kaxinawá) people, and by many other indigenous groups including the Amahuaca, Katukina, Kulina, Yawanawá, Matsés, Marubo and Mayoruna. It is still used widely amongst indigenous people in the Amazon to this day.
The first observations of Kambô use were made by a French priest, Father Constantin Tastevin in 1925 whilst he was staying with the Huni Kuin (Kaxinawá) tribe in the upper Juruá River in Brazil. In the 1980’s an American Anthropologist, Katherine Milton described Kambô use among the Mayoruna tribe in Brazil and in the 1980’s Peter Gorman wrote about his experiences taking Kambô with the Matsés tribe in Peru.
During the 1990’s, rubber tappers in Brazil learned about Kambô from the Amazon Indians. They began to take it out into the towns of Acre and apply it themselves. Having spent several years living with the Katukina, Francisco Gomes from Cruzeiro do Sol was one of the first people to pioneer the use of Kambô outside the Amazon. The practice spread and soon people in the larger cities of Brazil were using Kambô.
What is Kambô?
The frog is nocturnal and arboreal and due to the fact that it has no natural predators is found in abundance across the Upper Amazon rainforest areas of Bolivia, Brazil, Colombia, Peru, French Guiana, Suriname and Venezuela. The only known threats to this species of frog at the moment are spawned predation and the potential destruction of their habitat.

They are large frogs, the male bodies being between 9-10 cm and the females 11-12cm. The dorsum is a vibrant green and the belly a creamy white. They have dark spots on the chest, flank and legs. Reproduction occurs throughout the year, peaking between November and May. They construct hanging nests from folded leaves 1-3 metres above ponds and streams. The females deposit a gelatinous mass containing their eggs into these nests. Theirs is the largest spawn found amongst arboreal frogs of the Amazon. A single spawn contains on average 1000 eggs from which tadpoles emerge within 11-14 days. No one is 100% certain what the catalyst for producing the secretion is, but it is widely believed to be sequestered from their diet. This is why the frogs do not produce their secretion when they are removed from their natural environment.
If done correctly the frogs have not harmed in any way when the Kambô is taken from them.
A traditional medicine in the Amazon, Kambo is the common name in South America for the secretion from the skin of Phyllomedusa bicolour, a tree frog that inhabits certain parts of the Amazon rainforest. The secretions are characteristic of the Phillomedusa family and have traditionally been used as medicine by indigenous tribes such as the Katukina, Yawanawa, Cashinahua and Matses. Traditionally, the purpose of this practice – usually named after the frog, Kambo or Sapo (the word for toad in Spanish) – is to initiate a deep cleansing of the body and soul, to cure the panema (which could be translated as “Bad Luck in the Hunt”), to give strength and cure other diseases.
Interest in the healing potential of traditional Amazonian plants and animals, such as Kambo, Ayahuasca or the secretions of the toad Bufo alvarius, among others, is growing in modern urban civilizations, probably due to a growing dissatisfaction with Western medicine that runs parallel to an equally growing interest in alternative medicines and Amazonian shamanism. In this context, it is important to raise awareness of the history and nature of these medicines, as well as the potential risks that may accompany improper use.
These aspects are important to consider when hoping to understand their healing potential and when trying to “do no harm” to both the native cultures and the individuals who engage in the practice.
Traditional use of Kambo in practice
Once captured, the frog is carefully tied into an X shape. The frog will then secrete secretions on its skin as a defense mechanism against predators. This stress-induced secretion is collected on a stick and left to dry for storage. For traditional cultures, this is a medicine that acts on the physiology of the organism and the body, and also on the area that is beyond the material, ie. Soul. Kambo is considered an entity or spirit that is responsible for the healing process. The frog is treated with respect and never harmed, because, according to Amazonian beliefs, harming it would anger the animal spirit and thus could not bring healing. After extraction, the frog is released back into the forest.
The application of the extracted poison is done by applying a superficial wound on the skin of the person being burned with a stick and placing the rehydrated secretion over the burn, which appears like a pea-sized spot on the skin. It is usually applied to the hands and feet. The effect depends on the number of dots but also on the freshness of the Kambo.
The usual minimum dose is about 1 to 3 drops, but the number can vary greatly depending on the needs of the user.
By applying Kambo to a fresh wound, it can be absorbed subcutaneously and enter the blood vessel system. Within a few minutes, an acute physiological response is manifested, which is usually characterized by increased heart rate, sweating, dizziness, and sometimes nausea and vomiting. The acute effect is attributed to the presence of peptides (chains of amino acids like proteins, but shorter in length) that last for several minutes before disappearing. After the effects wear off, traditional Kambo medicine is said to leave the individual feeling increased strength, sensory awareness, and mental clarity. The peptides present in Kambo do not produce any psychoactive effects.
Composition of Kambo
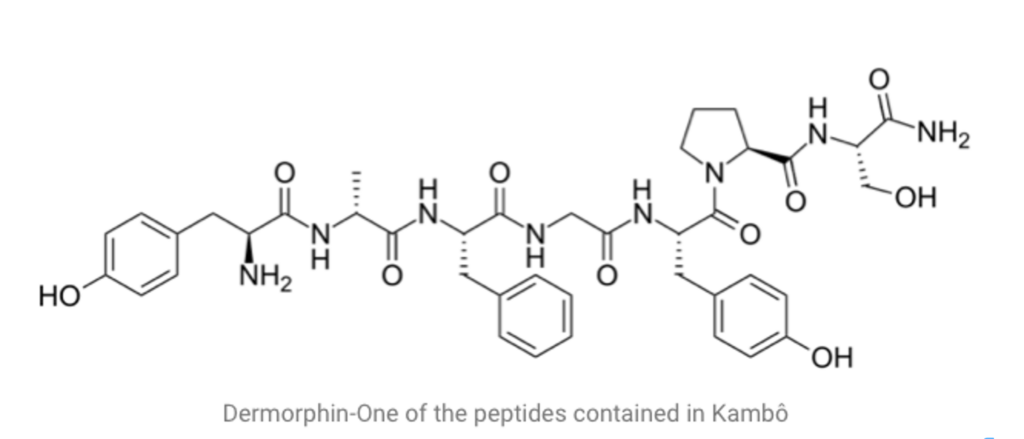
The secretion of Phyllomedusa bicolor contains a large number of bioactive peptides that are responsible for physiological effects. Some studies have performed several extraction methods to determine Kambo’s chemical composition and examine the effects of each peptide present in large amounts.
Composition order:
Phyllocaerulein is present in the highest concentration and appears responsible for Kambo’s main effects. It strongly affects the gastrointestinal smooth muscle, stimulating its motility, the flow of bile, pancreatic and stomach secretions, and analgesic effects in the central nervous system.
Phyllokinin has a hypotensive effect on the cardiovascular system.
Filomedusin also has strong hypotensive effects and stimulates intestinal motility (contributing to purification), and lacrimal and salivary secretions.
Sauvagin causes a drop in blood pressure due to vasodilatation of the mesenteric vascular area and causes intense tachycardia. It appears to activate the pituitary-adrenal axis in the central nervous system, causing an increase in plasma levels of corticosterone, catecholamines (like adrenaline) and glucose.
The opioid peptides, ala-deltorphin and lys7-dermorphin, have a strong affinity for opioid receptors that exceeds that of morphine receptors. This is one of the reasons why poison effects have been attributed to this substance. However, the concentrations present in Kambo are so low that they appear to have no significant biological activity in humans.
Peptides from the dermaseptin family were detected in reduced amounts. They are said to inhibit the growth of a wide range of microorganisms (protozoa, fungi, bacteria and viruses) without harming differentiated mammalian cells, and are therefore responsible for potential antibiotic activity.
The indigenous people have been using Kambo for centuries to heal and cleanse the body, strengthening its natural defences against bad luck. It is also believed to increase endurance and strength in hunting.
Today, shamans and naturopathic practitioners still use it to cleanse the body of toxins, as well as to treat numerous health conditions.
The latest scientific studies point to its ability to treat infections, regulate blood pressure, and have powerful pain-relieving effects. There is also evidence pointing to its effectiveness in the treatment of Alzheimer’s and Parkinson’s disease, depression, migraine, blood circulation problems, cancer, fertility, AIDS and hepatitis… It is no coincidence that several pharmaceutical companies have been researching the medicinal properties of Kamba for the past 25 years, and so far they have 27 patents that are based on the peptides that exist in Kambo.
Antimicrobial peptides from Phyllomedusa frogs
from biomolecular diversity to potential nanotechnologic medical applications
Screening for new bioactive peptides in South American anurans has been pioneered in frogs of the genus Phyllomedusa. All frogs of this genus have venomous skin secretions, i.e., a complex mixture of bioactive peptides against potential predators and pathogens that presumably evolved in a scenario of predator–prey interaction and defence against microbial invasion. For every new anuran species studied new peptides are found, with homologies to hormones, neurotransmitters, antimicrobials, and several other peptides with unknown biological activity. From Vittorio Erspamer’s findings, this genus has been reported as a ‘‘treasure store’’ of bioactive peptides, and several groups focus their research on these species. From 1966 to 2009, more than 200 peptide sequences from different Phyllo- medusa species were deposited in UniProt and other databases. During the last decade, the emergence of high-throughput molecular technologies involving de novo peptide sequencing via tandem mass spectrometry, cDNA cloning, pharmacological screening, and surface plasmon resonance applied to peptide discovery, led to fast struc- tural data acquisition and the generation of peptide molecular libraries. Research groups on bioactive peptides in Brazil using these new technologies, accounted for the exponential increase of new molecules described in the last decade, much higher than in any previous decades. Recently, these secretions were also reported as a rich source of multiple antimicrobial peptides effective against multidrug resistant strains of bacteria, fungi, protozoa, and viruses, providing instructive lessons for the development of new and more efficient nanotechnological-based therapies for infectious diseases treatment. Therefore, novel drugs arising from the identification and analysis of bioactive peptides from South American anuran biodiversity have a promising future role in nanobiotechnology.
Peptides of Phyllomedusa skin secretions
In spite of the large number of anuran species from different genera found within South America, a great deal of attention is being paid to the study of neotropical hylid frogs that belong to the subfamily Phyllomedusinae, as an excellent source of these molecules. Erspamer et al. (1985) also stated that ‘‘No other amphibian skin can compete with that of the Phyllomedusae’’. The initial efforts on Phyllomedusa skin secretions by Vittorio Erspamer followed by other scientists around the world during the last four decades revealed a complex cocktail of biologically active peptides with anti- microbial, hormonal, and neuro activities (Bevins and Zasloff 1990; Amiche et al. 1993). The peptides secreted differ sig- nificantly among species within this genus leading to an interesting molecular diversity, associated with possible specific differences present in the specie niche, such as the interactions with environment, predators, and pathogens characterizing Phyllomedusa species evolution.
The first peptide isolated from the Phyllomedusa skin was Phyllokinin [RPPGFSPFRIY], a bradykinyl-isoleucyl- tyrosine O-sulfate from P. rohdei in 1966 (Anastasi et al. 1966), followed by Phyllocaerulein [QEYTGWMDF-NH2] a cerulein-like nonapeptide from P. sauvagii in 1969 (Anastasi et al. 1969). All these bioactive peptides were discovered by Erspamer’s research group. Due to technical limitations, large numbers of specimens have to be killed in order to isolate, characterize, and perform the biological assays on the two peptides. Since the 1960s, the number of Phyllomedusa peptides discovered has increased expo- nentially (Fig. 1, inset) followed by the drastic reduction of specimens required for the analyses. Nowadays, it is pos- sible to carry out transcriptome analysis to build a cDNA library only with the secretions from a single living spec- imen (Chen et al. 2003b). The impacts caused by the bio- prospecting activity on the frog natural populations tend to zero through the development of non-invasive techniques largely due to scientific and technical advances.
The emergence of modern high-throughput molecular technologies involving de novo peptide sequencing via tandem mass spectrometry, cDNA cloning, and pharmacological screening applied to peptide discovery allowed fast structural data analysis and the generation of peptide sequence libraries, which in turn increased the capacity of peptide characterisation, remarkably reducing the number of samples needed (Shaw 2009).
The chronology related to the analyses of the Phyllo- medusa peptide discovery (Fig. 1) was impacted by the technological evolution applied to the study of venom- derived peptides, including the emergence of new research groups dedicated to the characterization of anuran venoms.
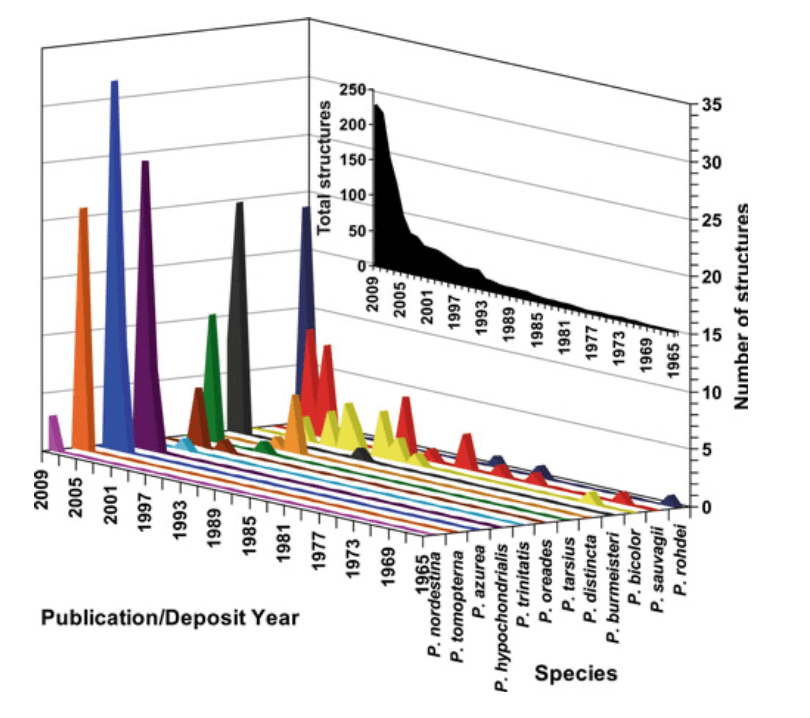
From 1966 to 2009, 227 peptide amino acid sequences, including peptide precursor cDNA sequences, belonging to the frog skin active peptide (FSAP) family from the skin of Phyllomedusa species (Fig. 1, inset) were published in scientific papers and/or deposited on genomic and/or pro- teomic data banks as the Universal Protein Resource Consortium (UniProt). The species P. azurea, P. bicolor, P. burmeisteri, P. distincta, P. hypochondrialis, P. nordestina, P. oreades, P. rohdei, P. sauvagii, P. tarsius, P. tomopterna, and P. trinitatis that belong to all groups, except the P. perinesos group, had their secreted peptides sequenced by 2009 (Table 1).
The Phyllomedusa skin peptides are grouped into three main groups according to their ‘‘primary’’ activity: anti-microbial peptides (AMPs); smooth muscle active peptides; and nervous system active peptides (Table 2) (Erspamer et al. 1981). However, peptides’ secondary activities were not considered in this systematization. The first group acts as a skin anti-infective passive defence barrier, and the second and the third groups cause the disruption of the predator homeostasis balance. The biological activity of the hypnotic peptides is still unknown.
Antitumor and Angiostatic Activities of the Antimicrobial Peptide Dermaseptin B2
Cancer is a disease that nowadays seems to become the most important cause of death. Nevertheless, in the last few decades significant progresses has been made in terms of understanding and treatment of this disease. Depending on the diagnosis, origin and state of the cancer several managing options exist, including surgery, radiation therapy, and chemotherapy. Unfortunately, concerning chemotherapy, the activity of most chemotherapeutic agents counteract also against the healthy fast-dividing cells of the body, such as blood cells and the cells lining the mouth, stomach, and intestines. Furthermore, they can depress the immune system and cancer cells frequently develop resistance to many anti-cancer drugs that greatly reduce their therapeutic usefulness. Other common side effects include fatigue, the tendency to bleed easily, gastrointestinal distress, rapid weight loss, or occasionally weight gain, and temporary hair loss.
Due to the constant need to improve or find new therapeutic agents against cancer and especially those that are able to evade drug resistance and other significant side effects, peptides have become one of the new research targets. A group of interesting natural-source peptides consists of antimicrobial peptides (AMPs). They have been isolated from a wide variety of organisms as single-celled microorganisms, insects, plants, birds, fish, amphibians, and mammals, including humans. AMPs were initially discovered due to their role in the clearance of microorganisms and are released in response to infection by a different regulatory process. AMPs mainly target the plasma membrane and they quickly kill the microorganisms in vitro (within a few minutes). This killing activity covers a wide spectrum including Gram-positive and -negative bacteria, fungi and protozoa without being cytotoxic to mammal cells at the doses that have shown antimicrobial activity. Besides, AMPs kill pathogens that are known to be multi-resistant to conventional antibiotics and since it is very difficult for a microorganism to radically change the organisation of its plasma membrane, potentially low levels of induced resistance occur compared to conventional antibiotics.
Interestingly, in addition to the above-named activities, a growing number of studies showed that some of the cationic AMPs exhibit a broad spectrum of cytotoxic activity against cancer cells. AMPs that are able to kill cancer cells can be placed into two categories: the first contains AMPs that are highly potent against bacteria and cancer cells but not against normal mammalian cells, for example, insect cecropias and magainins. The second group is composed of AMPs that are cytotoxic for bacteria, cancer cells, and normal mammalian cells. Some examples of this last group include the bee venom melittin [21], tachypneic-II isolated from the horseshoe crab, human neutrophil defences, and human LL-37. Nevertheless, many AMPs do not possess any anticancer activity.
Recently, we have reported significant antitumor activity of the AMPs derma septin (Drs) B2 and Drs B3 against human cancer prostate cells PC3 in vitro. Drs B2 and Drs B3 are members of the Drs B family and are derived from the skin secretions of the South American frog Phyllomedusa bicolour. They have high membrane-lytic antibacterial activity whereby they quickly kill Gram-positive and Gram-negative bacteria, yeast, protozoa and filamentous fungi, and have little or no haemolytic activity. As Drs B2 is regarded as the most abundant member and the most active peptide of the B family with a minimal inhibitory concentration in the micromolar range we further investigated the antitumor activity of this peptide. Drs B2, also known as adenoregulin, is an a-helical amphipathic polycationic polypeptide (NH2-GLWSKIKEVGKEAAKAAA- KAAGKAALGAVSEAV-CONH2) with a molecular mass of 3180 Da.
The aim of this study was to analyze the antitumor efficacy of Drs B2 on a wide range of human tumor cells in vitro and to evaluate its antitumor activity in a PC3 tumor xenograft mice model. In order to explore the possible mechanism of action of Drs B2 on tumor PC3 cells, experiments related to cell viability, cell death, membrane and/or mitochondrial integrity are performed. Additionally, immunostaining experiments using an anti-Drs B2 polyclonal antibody are accomplished to localize where Drs B2 is acting on and eventually inside the cells.
Results
Effect of Drs B2 on proliferation of tumor and non tumor cells
Several concentrations of Drs B2 were tested on the prolifer- ation of various tumor and non tumor cells from human origin. The dose-dependent activity of Drs B2 on cell viability curves is resumed in Table 1. Results are expressed in growth inhibition 50% (GI50) which indicates the peptide concentration that inhibit 50% of the cell growth. The data reveal that Drs B2 is most active against the proliferation of tumor adherent and non-adherent cell lines and shows GI50 values in the low micromolar range. The highest observed GI50 value of 8 mM is related to the inhibition of breast carcinoma MDA-MB231 cells after treatment with Drs B2. Also the proliferation of the non tumor but immortalized cells LB- EBV, HTK and PNT1A was inhibited by this peptide. At a maximum tested concentration of 15 mM, Drs B2 did not affect proliferation of the tested human primary normal cells G1947 and FD.
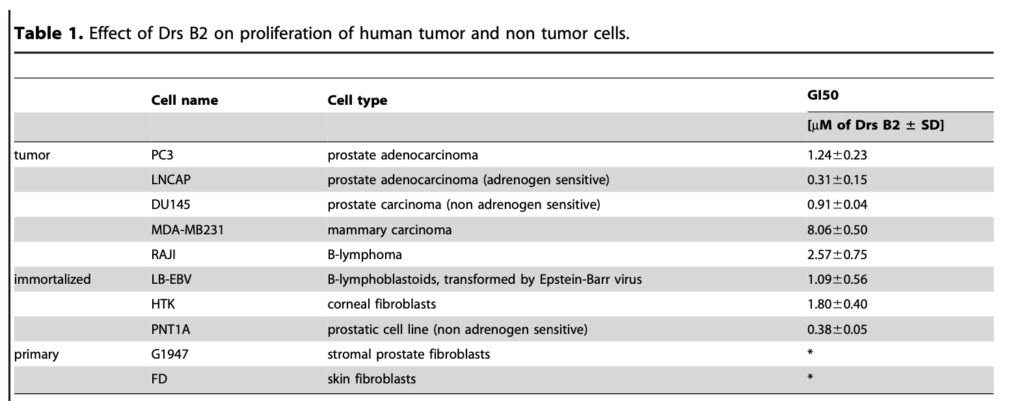
*No cytotoxicity was observed when cells were treated with a maximal concentration of 15 mM of Drs B2.
Effects of Drs B2 on PC3 and MDA-MB231 colony formation in vitro
Since Drs B2 showed a lower GI50 on tumor cells than on non tumor cells, Drs B2 was further studied to explore its activity against cancer cells and on colony formation of PC3 and MDA- MB231 cells in vitro. As shown in Figures 1A and 1B, treatment with Drs B2 of 0.1 mM significantly inhibited PC3 cell colony formation with more than 70% compared to untreated conditions. Concentrations above 2.5 mM showed a complete inhibition of colony formation. Similarly, MDA-MB231 colony formation was inhibited by Drs B2 (Figure 1C). Treatment with 1 mM inhibited colony formation of about 50% compared to untreated cells. Colony formation was completely inhibited when treated with 2.5 mM or higher. These experiments confirmed the anti- proliferative activity of Drs B2 on the PC3 and MDA-MB231 human tumor cell lines.
Effects of Drs B2 on endothelial cell proliferation and differentiation in vitro
Since we previously reported that partially purified peptides fractions from the skin secretions of the frog Phyllomedusa bicolour inhibited the proliferation and differentiation of ABAE cells, the effect of Drs B2 on the human endothelial cell was also explored. As shown respectively in Figures 2A and D, the proliferation of the HUVEC and ABAE cells was inhibited by Drs B2 in a dose-dependent manner. The angiostatic abilities of Drs B2 were observed by testing its effect on two in vitro angiogenesis models using ABAE cells on collagen according to Montesano and using HUVEC on Matrigel TM. In control conditions in the presence of FGF-2, HUVEC cells have formed a complete linked capillary network onto MatrigelTM 24 hours after plating (Figures 2B and C). Compared to the untreated control conditions, treatment with Drs B2 inhibited HUVEC pseudo capillary formation at 24 hours after treatment in a dose-dependent manner from 1 to 5 mM. These results are confirmed by the anti-angiogenic activity of Drs B2 on ABAE differentiation on collagen according to the Montesano model (Figures 2E and F). In the presence of FGF-2 ABAE cells formed capillary tubes onto collagen 4 days after plating whereas treatment with 5 mM Drs B2 completely inhibited the formation of these tubes (Figure 2F). Observations of both endothelial cell differentiation models suggest that Drs B2 disturbs the formation of capillaries without having toxic activity towards undifferentiated cells attached onto the layers.
Effects of Drs B2 on tumour growth in vivo
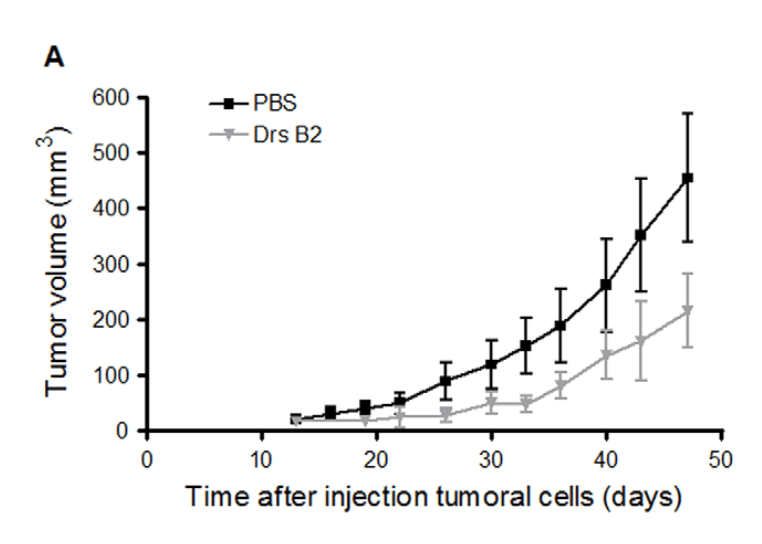
As shown in Figure 1, Drs B2 has the capacity to completely inhibit the colony formation of MDA-MB231 and PC3 cells in vitro. Since PC3 cells present an average sensibility to Drs B2 and are able to form tumours in mice, these prostate adenocarcinoma cells were chosen as the representative cell line for the remainder of this investigation. Consequently, the effect of Drs B2 was evaluated in vivo via a xenograft model in nude mice using this type of human tumour cells. Thirteen days after the injection of PC3 cells one single tumour of around 25 mm3 was developed where after treatments were started. Figure 3A shows the tumour volume versus the time of treatment. At the end of the experiment, Drs B2 inhibited tumour growth by more than 50% compared to tumours treated with PBS. However, due to the large dispersion of the tumour volume in mice from each group, no significance was obtained when using the two-tailed t-test. In two mice, Drs B2 completely inhibited tumour growth. The inhibitory patron of tumour weight due to Drs B2 was equal to that of the tumour size (Figure 3B). Still, for a similar reason, this inhibition was not significant when using the same statistical analysis. Moreover, compared to non-tumour-bearing mice, total blood cell count was not significantly changed when treated with Drs B2 (data not shown). Additionally, no mortality, long-lasting side effects, or signs of weakness, diarrhea, appetite, or lethargy were observed during this experiment.
In some cases, kambo can help with a number of conditions, including:
Addiction
Alzheimer’s disease
Anxiety
Some bring back cancer
Chronic pain
Depression
Diabetes
Hepatitis
HIV and AIDS
Certain infections
Infertility
Rheumatism
What is the procedure?
The first part of the procedure involves drinking 2 to 3 liters of water.
Next, the practitioner will use the stick to make a series of small burns on the skin (gates). Then Kambo is applied to them.
From the gates, Kambo enters the lymphatic system and the bloodstream. This usually results in an increased pulse, pulsation of body parts, and increased temperature, but also symptoms similar to increased blood pressure, these symptoms last for a short time and go quickly before vomiting itself, which leaves a feeling of satisfaction and relief that remains even after the cleansing process itself.
Each tribe has its own legend or story about how it started using Kambo. The most widespread legend comes from Brazil from the Kaxianawá tribe of the northern Amazon region of Brazil. This legend of Kaxinawa says that the Indians of the tribe were very sick and that their doctor / Pajé tried to cure them all. Nothing helped.
On the Ayahuasca journey, he entered the forest and was visited by the spirit of the jungle. He brought in his hands a frog from which he took the white secretion and taught Pajé how to apply it. Returning to the tribe and following the guidance he was given, Pajé was able to heal his siblings. From then on it was known as Pajé Kampu or Kampum.
When he died, his spirit lived in the frog, where it continued its mission to protect the health of those who defend the forest. The medicine became known as Kambo, but in some tribes, it is called Sapo, Dow-Kiet, Kampu or Vacina da Floresta.
For thousands of years, Kambo has been used as medicine by the Kaxinawá people and many other indigenous groups, including the Amahuaca, Katukina, Kulina, Yawanawá, Matses, Marubo and Mayoruna. It is still widely used among the indigenous people of the Amazon to this day.
The first observations about the use of Kambo were made by a French priest, Father Constantin Tastevin, in 1925, while staying with the Kaxinawá tribe in the upper Juruá River in Brazil. In the 1980s, American anthropologist Katherine Milton described the use of Kambo among the Mayoruna tribe in Brazil, and in the 1980s, Peter Gorman wrote about his experiences taking Kambo with the Matses tribe in Peru.
In the 1990s people in Brazil learned about Kambo from the Amazonian Indians. They began to take it to the cities of Acre and apply it themselves. Having spent several years living with the Katukina, Francisco Gomes of Cruzeiro do Sol was one of the first to pioneer Kambo outside the Amazon. The practice spread and soon people in the major cities of Brazil were using Kambo.
Contraindications
The following may not safely take Kambô:
- People with serious heart problems
- People who have had a stroke
- People on medication for low blood pressure (this is extremely rare)
- People who’ve had a brain hemorrhage
- People who have aneurysms or blood clots
- People who lack the mental capacity to make the decision to take Kambo
- People with serious mental health problems excluding depression and anxiety
- People undergoing chemotherapy or radiotherapy for 4 – 6 weeks afterward
- People who take immune-suppressants for an organ transplant
- Women who are pregnant or may be so
- Women who are breast-feeding a child under 6 months old
- People with Addison’s disease
- People with Ehlers Danlos Syndrome
- People with current and severe Epilepsy
- Are recovering from a major surgical procedure
- Under 18
- Animals
Cautions
Caution is also required in the following cases:
- People taking immune-suppressants for auto immune disorders.
- Active drug or alcohol addiction.
- Long term or water fasting for 7 days before or after Kambo other than the required fasting.
- Colonics, Enemas, liver flushes or any water based detox should be avoided within 3 days either side of taking Kambo.
- If someone has taken Bufo 5-MeO-DMT there is a 6-8 week waiting period before Kambo.
Oesophagus/Esophagus Rupture
Because Kambo can cause violent vomiting we need to have some caution around certain conditions that could weaken the Oesophagus/Esophagus. These do not necessarily preclude you from experiencing Kambo but it is advisable to make sure to let your Practitioner know if you have been affected with any of the following:
- Boerhaave’s Syndrome (spontaneous rupture of the oesophagus)
- Severe injury, or trauma to the Oesophagus/Esophagus from endoscopy, or injury to the neck.
- Tumours, or Ulcers in the throat
- Those who have or have had bulimia
- Gastro-intestinal Reflux
- Chronic inflammatory response syndrome due to mold exposure
- Untreated eosinophilic esophagitis
- Oesophagus/Esophagus Varices
- Portal Hypertension
You’ve heard about this amazing frog secretion and all the benefits working with it can bring and now you want to discover it for yourself. A google search reveals over 1.5 million results for Kambo – an overwhelming amount of information. So how do you choose the right person? Here’s our checklist to help you.
- Ask them where and with whom they have trained. Different tribes and teachers’ work differently with Kambo. It’s good to ask them exactly how they work and what will happen during a session. Perhaps they have a website where you can read this information in advance.
- Ask how long they have been working with Kambo and how many people they have treated. This is definitely a treatment where experience or comprehensive training is beneficial to ensuring a good first experience.
- If you are seeking Kambo to work with a particular health condition ask if the practitioner has experience in treating that issue and it is within their area of competence.
- Safety – the Kambo experience can be intense. From investigating your proposed practitioner do you get a sense that you will be held in a safe space? Do they know how much water you should drink? Will they know how to handle it if you faint i.e. do they know how to put you into the recovery position. Do they know how much secretion is appropriate for you for a first time? Will they do a test point?
- Have you been provided with a full account of how to prepare for your session including information on fasting?
- Did the person ask your reason for wanting to experience Kambo? Will you be given the opportunity to discuss any private issues in advance of the ceremony?
- Kambo is not for everyone. Did they check your medical history when you booked and have they checked to see if you have any contraindications?
- Venue – ask about the facilities; number of bathrooms and are they close? – And what you need to bring with you. Some practitioners provide mattresses, blankets, buckets and water and some ask you to bring your own so make sure you know what to bring.
- What is the maximum number of people for the circle and how many assistants will be present? Ask if the assistants are trained in assisting specifically in Kambo circles. You can also ask if they provide private treatments if you feel more comfortable with that.
- Location – how far from home will you have to travel? Is there space for you to rest after the session if necessary?
- Ask about the style of the ceremony – some practitioners practice a shamanic ceremonial style and with others it will be more akin to visiting a treatment centre or clinic. Choose someone who’s style makes you feel comfortable.
Following this checklist will give you the best chance of experiencing a life-enhancing first experience with this wonderful secretion from the upper Amazon.
Ivan Nikitović
